Redefining Drug Delivery
Biotech simplifying chronic care, from startup to exit, phases of M&A, SaaS pricing, jobs, events, and more
Startup Pirate is a newsletter about entrepreneurship, technology, and startups. Made in Greece. If you haven’t subscribed, join 5,700 readers by clicking below:
Redefining Drug Delivery
The following is a conversation with Konstantinos Tzortzakis, co-founder and Chief Business Officer at Anodyne Nanotech, a biotech company that develops transdermal forms of breakthrough medicines. Anodyne’s technology is a drug delivery platform, the HeroPatch, which promises to simplify care for chronic diseases through small patches placed on top of the skin where the drug is absorbed into the bloodstream, using proprietary microneedle technology. We discuss:
where traditional drug delivery like injections or pills fall short
the promise of microneedles and therapies that could unlock
what's beneath a microneedle drug delivery patch
current state of development and next steps for Anodyne
what's in it for the pharma industry
Let's get to it.
Konstantine, it's great to have you here. What's the best way to describe the technology Anodyne is developing?
KT: Thanks Alex! The HeroPatch is a platform that allows existing drug products to be less expensive, less toxic, and more convenient to use. To better understand this, I'd love to take a step back first and share shortcomings of the available drug delivery methods. Typically, drugs are delivered to patients via subcutaneous injections, IV infusions (administered directly into a person's vein), and oral tablets. The problem is that these methods usually don't enable the full potential of compounds, especially for biologics (large molecules that typically require injections). This is evident for several diseases but mainly chronic ones (heart disease, diabetes, obesity, rheumatoid arthritis, etc) where people have to deal with daily or weekly injections.
Approximately 60% of adults in the US have a chronic condition, and yet only half of them take their medication as prescribed due to:
Need for refrigeration, which increases the supply chain cost and complexity, and further complicates patients’ regular activities (for example, travelling becomes very challenging).
Mental or physical discomfort associated with injections.
Side effects and potential toxicity from early spikes in blood concentration as the drug is delivered all at once.
Pills offer very low absorption from the body, leading to high variability in efficacy based on diet and higher doses of active pharmaceutical ingredient (further contributing to side effects).
Consequently, chronic care is very complex. The impact? More than one hundred thousand preventable deaths, over $500B in preventable healthcare costs, and over $600B in lost revenue for pharmaceuticals, every year!
That’s why we started Anodyne with my co-founders Jake and Hojat in 2019 to redefine drug delivery with a focus on chronic diseases. We have created a small patch, the HeroPatch, a drug delivery platform that leverages our proprietary microneedle technology to deliver clinically meaningful doses of biologics through the skin. This will give patients a superior alternative to injections.
Not only does it offer a more convenient alternative for the patient who requires daily or weekly injections, but also has the potential to reduce side effects by avoiding early spikes in blood concentration and costs by eliminating cold storage and shipping requirements, as the patch is stable at room temperature. We envision this as a new platform for chronic care.
The promise of microneedles in drug delivery has been discussed for a while now, right? I believe they were first mentioned in the literature decades ago, and since then, we can mainly find examples of limited applications. What makes Anodyne different?
KT: Microneedles have been around for decades, therefore medical device and pharmaceutical companies recognise their potential benefits. However, the limitations in drug loading and the complex and expensive manufacturing were the main limiting factors so far.
That's where we come in. Our main advantage is, in fact, drug loading. We can load multi-milligram doses of large and small molecules where most competitors are probably orders of magnitude less. Hence, we can deliver clinically relevant doses of therapeutics, not just vaccines or some very potent molecules. Our technology is also tailored to deliver a combination of multiple drugs within a single patch. The latter is one of our superpowers, as many of the most effective treatments for chronic diseases in the future will be combination therapies.
What are the limitations compared to the popular drug delivery methods like injections and tablet treatments?
KT: There is a dose limitation. We cannot deliver grams or hundreds of milligrams of molecules, hence, for cases that require very high doses, you probably have to take either an IV infusion or a subcutaneous injection. There are also treatments where instant delivery of the drug is required, e.g. allergic reactions, and for those, it's pretty well known that the transdermal route is not a great fit given their sustained pharmacokinetic profile. That's when injections are necessary. On the other hand, oral formulations work well for certain drugs with high absorption (typically small molecules which make up many common over-the-counter medications such as aspirin and painkillers or antibiotics) and low cost to manufacture. Therefore, there is no need for an alternative.
Please give us some ideas of the types of molecules microneedles can work with and therapies this could unlock.
KT: The great thing about developing a platform is that it can fit several use cases. Given the benefits we discussed earlier, our focus is on biologics. Imagine an entirely new and much more convenient method to treat type 2 diabetes and obesity with GLP-1RAs. Another example would be auto-immune diseases like rheumatoid arthritis with monoclonal antibodies such as adalimumab, as well as future applications with mRNAs, siRNAs, etc. There is a large pool of molecules, including small and large molecules, that we can support and offer a wide array of advantages in collaboration with our pharma partners.
At Anodyne, we primarily work with already-approved drugs for which pharmaceutical companies would like to offer an alternative delivery method or off-patent drugs (the patent has expired) that we can develop ourselves and subsequently partner with a pharmaceutical company for commercialisation.
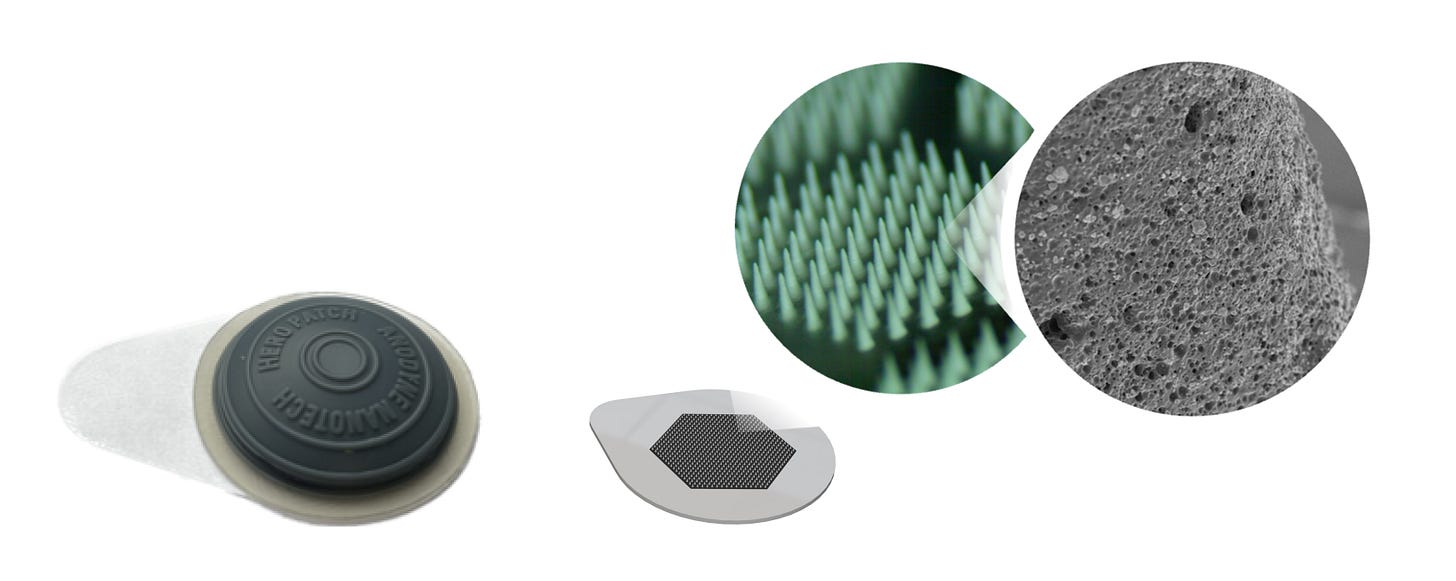
I'd love to geek out on the technical details of the patch and the materials you use.
KT: The primary differentiation in our microneedling technology is that we use the powder form of the drug. A solid-state drug is embedded directly throughout the entire porous structure of a hard polymer, resin, which can penetrate the skin and deliver the compound. That gives us the ability to leverage a significant part of the volume of a microneedle, resulting in high drug loading. This also provides room temperature stability, which eliminates the need for cold storage and shipping we previously discussed.
Once the patch is applied to the skin, the microneedles are surrounded by the epidermal and dermal space, where the interstitial fluid reconstitutes the dry formulation, and the active pharmaceutical ingredient diffuses out of the polymeric matrix. After the delivery of the active pharmaceutical ingredient, the microneedles remain intact, so the patch is removed and discarded. That's the core of it, and of course, we can adjust the form factor to accommodate any drug particularities.
What’s in it for the pharma industry?
KT: Pharmaceutical companies can convert more patients to the drug, increasing revenues and market share. For medicines that are still on patent, there is also potential for patent extension because it's a new technology and route of administration. This will help revive the IP of many pharma companies. Moreover, Anodyne helps them significantly decrease costs, reduce plastic use, and meet their carbon footprint targets, given we eliminate the need for cold chain distribution.
Last but not least, the fact that we can load multiple active pharmaceutical ingredients in a single patch will unlock new treatments through combination therapies, which otherwise would require multiple injections or years of formulation work in a more complicated device.
What's the current state of development, and what are the next steps for Anodyne? Anything that you can share in public, of course :)
KT: We recently derisked our technology platform in a human study. Now we are focusing on product development activities for specific therapeutic programs. As you might imagine, this progress has attracted the attention of some of the largest pharma organisations, and we look forward to advancing programs with our current and future partners. We are also making significant investments in automation and manufacturing to support future clinical development.
Thank you so much for taking the time, Konstantine!
KT: It was a pleasure.
Jobs
Check out job openings here from startups hiring in Greece.
News
Athos Therapeutics secured $35m to revolutionize autoimmune disease and cancer treatment with AI. (link)
Cloud kitchen company StiQ raised €10m to scale operations in Greece. (link)
finloup, a company that enables leasing tech products for businesses, raises over €1m from Velocity Partners, Genesis Ventures, and other investors. (link)
Didimi, a digital twins and information management platform for construction, raised €880k Pre Seed. (link)
A new VC fund based in Thessaloniki, Loggerhead Venture Fund, with a size of €10m to invest in early-stage Greek startups. (link)
Wrap up and participating teams from the investor day at Egg incubator. (link)
Greece set to acquire a CubeSat constellation with a budget of €60m. (link)
Resources
Reputation for Ethereum validators, conversational AI, and the story of maritime AI startup Deepsea from startup to exit in the latest Open Coffee Athens. (link)
Running strong reference checks in hiring by Dimitris Glezos, founder of Transifex. (link)
The three phases of M&A process and how long they can take by Alex Loukas, Managing Director at GrowthPoint. (link)
SaaS pricing: what I want vs what I offer by Lambros Petrou, Senior Software Engineer at Datadog. (link)
Antonis Kalipetis and Paris Kasidiaris from Mikri Kouventa discuss the state of cloud. (link)
Spending time managing product delivery as a Product Manager by Manos Kyriakakis, Product Lead at Skroutz. (link)
Skroutz: a year in review from George Hadjigeorgiou, co-founder & CEO at Skroutz. (link)
Events
Hosting San Francisco Greeks In Tech on Mar 13 - join us! More details here.
Uncharted Territories in Mobile Application Testing by Athens SDET on Feb 26
Cross-functional collaboration in creating the Roadmap by Product Community Greece on Feb 29
Open Coffee Thessaloniki #81 by Open Coffee on Feb 29
Clojure 101: Interactive Introduction by Thessaloniki not-only Java on Feb 29
Angular Athens 21th Meetup by Angular Athens on Mar 5
Scale-up Greece with Daniel Isenberg by Scale-up Greece on Mar 7
Impact the Future by Women Techmakers Greece on Mar 9
Microsoft for Startups Discovery Day by Microsoft Greece on Mar 13
That’s all for this week. Tap the heart ❤️ below if you liked this piece — it helps me understand which themes you like best and what I should do more.
Find me on LinkedIn or Twitter.
Thanks for reading,
Alex